Personalized Medicine: The Future of Biotechnology and Genetic Engineering
Introduction: Healthcare Reimagined for the Individual
Imagine a world where your medical treatment is as unique as your fingerprint—where medications are precisely tailored to your genetic makeup, where diseases are detected years before symptoms appear, and where prevention strategies are customized to your specific risk profile. This isn’t science fiction; it’s the emerging reality of personalized medicine, one of the most promising frontiers in biotechnology and genetic engineering.
For centuries, medicine has relied on a one-size-fits-all approach, with treatments designed for the “average patient.” Yet this approach ignores a fundamental truth: each person’s genetic makeup, environment, and lifestyle are unique, causing significant variations in how individuals respond to diseases and treatments. Personalized medicine (also called precision medicine) represents a paradigm shift away from this traditional model toward healthcare tailored to the individual.
$

In this comprehensive guide, we’ll explore how personalized medicine is transforming healthcare through biotechnology and genetic engineering, examine groundbreaking applications, address ethical questions, and look toward the remarkable future this field promises to create.
What Are Biotechnology and Genetic Engineering?
Biotechnology Defined
Biotechnology refers to the use of biological systems, living organisms, or derivatives thereof to develop products and technologies that improve our lives and the health of our planet. This interdisciplinary field merges biology with technology to harness cellular and biomolecular processes for practical applications.
Biotechnology spans several sectors:
- Red biotechnology focuses on medical applications, including personalized medicine
- Green biotechnology addresses agricultural and environmental applications
- White biotechnology involves industrial processes and biomanufacturing
- Blue biotechnology explores marine and aquatic applications
The foundation of modern biotechnology lies in our growing ability to understand and manipulate the fundamental building blocks of life, providing the tools needed for personalized medicine approaches.
Genetic Engineering Explained
Genetic engineering involves directly manipulating an organism’s genetic material using biotechnology. By altering DNA—the blueprint of life—scientists can modify gene expression, add new genes, or correct faulty ones.
Key genetic engineering techniques include:
- Recombinant DNA technology – Combining DNA from different sources
- Gene addition – Inserting functional genes to replace defective ones
- Gene silencing – Reducing or preventing the expression of specific genes
- Gene editing – Making precise changes to DNA using tools like CRISPR
These technologies form the foundation of many personalized medicine approaches, particularly those targeting genetic causes of disease.
The Personalized Medicine Revolution
What is Personalized Medicine?
Personalized medicine is an approach that tailors prevention, diagnosis, and treatment strategies to the individual characteristics of each patient. Unlike traditional medicine, which applies standardized treatments based on broad disease categories, personalized medicine considers:
- Genetic variations that affect disease risk and treatment response
- Lifestyle factors that interact with genetic predispositions
- Environmental exposures that influence health outcomes
- Molecular characteristics of diseases, particularly cancer
- Microbiome composition and its impact on health
The core principle is simple yet revolutionary: by understanding what makes each patient unique, we can provide more effective, efficient, and safer healthcare.
$
*** QuickLaTeX cannot compile formula:
\begin{tikzpicture} \begin{tikzpicture}[mindmap, concept color=blue!40, level 1 concept/.append style={sibling angle=72}] \node [concept] {Personalized\Medicine} child [concept color=green!50] { node [concept] {Right Treatment} } child [concept color=red!50] { node [concept] {Right Patient} } child [concept color=purple!50] { node [concept] {Right Time} } child [concept color=orange!50] { node [concept] {Right Dose} } child [concept color=teal!50] { node [concept] {Right Approach} }; \end{tikzpicture}
*** Error message:
TeX capacity exceeded, sorry [grouping levels=255].
leading text: ...style={sibling angle=72}] \node [concept] {
\end{tikzpicture}$Core Technologies Enabling Personalized Medicine
Several biotechnological advances have made personalized medicine possible:
Genomic Sequencing
The ability to read an individual’s genetic code:
- Whole genome sequencing – Reading all 3 billion DNA base pairs
- Whole exome sequencing – Focusing on protein-coding regions
- Targeted sequencing – Analyzing specific genes of interest
- Pharmacogenomic panels – Identifying variants affecting drug response
As sequencing costs have plummeted from billions to hundreds of dollars, genetic information has become increasingly accessible for clinical use.
Multi-Omics Technologies
Looking beyond DNA to capture the full picture of health:
- Transcriptomics – Analyzing gene expression patterns (RNA)
- Proteomics – Studying protein levels and modifications
- Metabolomics – Measuring small molecule metabolites
- Epigenomics – Examining modifications that affect gene expression
- Microbiomics – Characterizing microbial communities
These technologies provide dynamic information that complements the static blueprint of DNA.
Advanced Biomarker Testing
Detecting biological indicators of health states:
- Liquid biopsies – Finding cancer DNA in blood samples
- Immune profiling – Characterizing immune system components
- Molecular imaging – Visualizing specific molecular targets
- Digital biomarkers – Health data from wearable devices
Computational Tools
Making sense of complex biological data:
- Artificial intelligence – Pattern recognition in large datasets
- Machine learning – Predictive modeling for disease risk and progression
- Systems biology – Understanding biological networks holistically
- Bioinformatics – Specialized analysis of biological information
How Personalized Medicine is Revolutionizing Various Industries
Healthcare Transformation
Personalized medicine is fundamentally changing medical practice:
Oncology Revolution
Cancer treatment has been transformed by precision approaches:
- Tumor genomic profiling has replaced treatment based solely on cancer location
- Targeted therapies address specific molecular changes in tumors
- Immunotherapies are matched to patients most likely to respond
- Treatment monitoring uses circulating tumor DNA to detect resistance early
This approach has dramatically improved outcomes for many cancer patients, turning some previously fatal diagnoses into manageable conditions.
$
*** QuickLaTeX cannot compile formula:
\begin{tikzpicture} \begin{axis}[ title={5-Year Survival Rates for Selected Cancers}, xlabel={Treatment Approach}, ylabel={5-Year Survival Rate (%)}, ymin=0, ymax=100, symbolic x coords={Traditional, Targeted, Immunotherapy, Combination}, xtick=data, legend pos=north west, ymajorgrids=true, grid style=dashed, width=12cm, height=8cm, ]\addplot[ color=blue, mark=square, ] coordinates { (Traditional,25) (Targeted,45) (Immunotherapy,52) (Combination,65) }; \addlegendentry{Metastatic Melanoma}\addplot[ color=red, mark=triangle, ] coordinates { (Traditional,18) (Targeted,40) (Immunotherapy,35) (Combination,50) }; \addlegendentry{Non-Small Cell Lung Cancer}\addplot[ color=green, mark=o, ] coordinates { (Traditional,30) (Targeted,60) (Immunotherapy,48) (Combination,72) }; \addlegendentry{Certain Leukemias}\end{axis} \end{tikzpicture}
*** Error message:
File ended while scanning use of \pgfplots@@environment@axis.
Missing $ inserted.
Emergency stop.
$Rare Disease Diagnosis
Ending diagnostic odysseys for patients with uncommon conditions:
- Whole genome/exome sequencing can identify the cause of mysterious symptoms
- Treatment pathways can be developed based on specific genetic variants
- Patient matching connects individuals with similar rare genetic conditions
- Targeted drug development addresses previously untreatable disorders
Pharmacogenomics Implementation
Matching medications to genetic profiles:
- Dosing guidance based on metabolism genes
- Adverse reaction prevention by identifying genetic risk factors
- Efficacy prediction to select treatments most likely to work
- Polypharmacy management to prevent drug interactions
More than 200 FDA-approved medications now include pharmacogenomic information on their labels.
Pharmaceutical Industry Evolution
Personalized medicine is transforming drug development:
Target Discovery Revolution
Finding the right disease mechanisms to address:
- Genetic basis identification for complex diseases
- Patient stratification into molecular subgroups
- Biomarker development for tracking treatment response
- Novel pathway discovery through network analysis
Clinical Trial Transformation
Changing how new treatments are tested:
- Basket trials test drugs across cancer types based on shared mutations
- Adaptive designs modify protocols based on emerging data
- N-of-1 trials treat each patient as their own control
- Enrichment strategies select patients most likely to benefit
Companion Diagnostics
Tests that determine which patients should receive specific therapies:
- Co-development of tests alongside medications
- Regulatory requirement for many targeted therapies
- Reimbursement leverage demonstrating value through precision
Preventive Healthcare Approaches
Moving from treatment to prediction and prevention:
Genetic Risk Assessment
Identifying predispositions before disease occurs:
- Polygenic risk scores combining effects of many genetic variants
- Actionable risk factors with prevention strategies
- Cascade testing for family members when heritable risks are found
- Periodic reassessment as understanding of genetics improves
Lifestyle Optimization
Personalized wellness based on individual biology:
- Nutrigenomics tailoring nutrition to genetic makeup
- Exercise response prediction based on genetic factors
- Sleep optimization accounting for chronotype genetics
- Stress management strategies matched to personal physiology
Recent Breakthroughs in Genetic Engineering for Personalized Medicine
CRISPR and Gene Therapy Advances
Cutting-edge technologies enabling precision treatments:
In Vivo Gene Editing
Editing genes directly in the body:
- Eye disorders: Direct injection of CRISPR components
- Liver-targeted approaches: Using lipid nanoparticles
- Central nervous system delivery: Addressing neurological conditions
Ex Vivo Cell Engineering
Modifying cells outside the body:
- CAR-T cell therapy: Engineering immune cells to fight cancer
- Gene-corrected stem cells: For blood disorders like sickle cell disease
- Induced pluripotent stem cells: Patient-specific regenerative medicine
Next-Generation Gene Editing
Beyond traditional CRISPR approaches:
- Base editing: Making single nucleotide changes without double-strand breaks
- Prime editing: Precise “search and replace” editing
- RNA editing: Temporary modifications without altering DNA
Multi-Omic Integration
Combining different layers of biological information:
| Omic Layer | What It Measures | Personalized Medicine Application |
|---|---|---|
| Genomics | DNA sequence | Disease risk, drug response |
| Transcriptomics | Gene expression (RNA) | Disease activity, treatment response |
| Proteomics | Protein levels and modifications | Functional changes, biomarkers |
| Metabolomics | Small molecules/metabolites | Metabolic state, drug effects |
| Epigenomics | DNA/histone modifications | Environmental impacts, aging |
| Microbiomics | Microbial communities | Digestive health, immunity, response to therapy |
Integration of these data types provides a more complete picture of individual health than any single approach.
Digital Health Integration
Merging biotechnology with digital technology:
Continuous Monitoring Technologies
Real-time health data collection:
- Wearable biosensors tracking physiological parameters
- Implantable monitors for chronic conditions
- Digital biomarkers correlating with disease states
- Remote patient monitoring feeding into personalized algorithms
AI-Driven Decision Support
Computational assistance for healthcare providers:
- Treatment recommendation engines synthesizing complex data
- Pattern recognition identifying subtle disease manifestations
- Predictive algorithms for disease trajectory
- Medication optimization accounting for multiple factors
Ethical Concerns and Controversies
Privacy and Data Security
Managing sensitive personal health information:
Genetic Privacy Challenges
Protecting the most personal data:
- Re-identification risk despite anonymization
- Secondary use concerns for research data
- Incidental findings unrelated to initial testing
- Family implications when genetic information is shared
Data Ownership Questions
Determining who controls health information:
- Patient rights versus researcher/company interests
- Commercialization of personal biological data
- Consent models for ongoing and future use
- Cross-border considerations with varying regulations
$
![]()
Access and Equity Issues
Addressing disparities in personalized medicine:
Economic Barriers
Challenges to equitable implementation:
- Cost of advanced testing and targeted therapies
- Insurance coverage variations for precision approaches
- Global healthcare disparities limiting access
- Infrastructure requirements for implementation
Representation in Research
Ensuring diversity in foundational data:
- Genomic database bias toward European ancestry
- Clinical trial enrollment disparities affecting evidence base
- Reference genome limitations for global populations
- Algorithmic fairness when developing prediction tools
Bioethical Considerations
Navigating complex moral questions:
Incidental Findings Management
Handling unexpected genetic discoveries:
- Clinical actionability determining what to report
- Right not to know versus potential benefit
- Pediatric considerations for adult-onset conditions
- Family notification when findings affect relatives
Therapeutic Access Decisions
Determining who receives limited resources:
- Cost-benefit assessment for extremely expensive therapies
- Orphan disease treatment development incentives
- Value-based frameworks accounting for precision
- Lottery systems when resources are constrained
Future of Personalized Medicine: Trends and Innovations
Emerging Technologies
Cutting-edge approaches expanding capabilities:
Spatial Omics
Adding dimensional information to biological data:
- Spatial transcriptomics mapping gene expression within tissues
- In situ sequencing reading genetic information directly in cells
- Multiplexed protein imaging visualizing many proteins simultaneously
- Single-cell spatial analysis understanding cellular neighborhoods
Genome Writing and Synthesis
Moving beyond editing to creation:
- Synthetic biology applications in cell therapy
- Gene circuit design for programmed cellular responses
- Minimal genomes determining essential genetic elements
- DNA data storage using genomic technology for information
Quantum Biosensing
Next-generation detection methods:
- Ultra-sensitive molecular detection at quantum scales
- Non-invasive monitoring through advanced sensors
- Field-deployable diagnostics for point-of-care precision
- Real-time molecular tracking in living systems
Convergence with Other Fields
Cross-disciplinary synergies:
AI and Advanced Computing
Computational approaches enhancing personalized medicine:
- Deep learning diagnostics outperforming human experts
- Federated learning preserving privacy while accessing diverse data
- Digital twin development simulating individual health systems
- Quantum computing applications for complex biological modeling
Nanotechnology Integration
Precise intervention at the molecular scale:
- Targeted drug delivery to specific cells or tissues
- Nanorobotics for minimally invasive procedures
- Molecular machines performing therapeutic functions
- Bioelectronic interfaces connecting technology with biology
Next-Generation Manufacturing
New approaches to producing personalized therapies:
- Distributed manufacturing closer to point of care
- 3D bioprinting of tissues and organoids
- Automated cell therapy production reducing costs
- Closed-system processing improving safety and efficiency
Future Applications
Transformative possibilities on the horizon:
Regenerative Medicine Personalization
Rebuilding tissues and organs:
- Patient-specific organoids for testing and transplantation
- Cell therapy optimization based on individual genetics
- Tissue engineering matched to recipient biology
- Aging reversal interventions tailored to personal epigenetics
Preventive Genomic Medicine
Proactive health management:
- Lifespan genomic screening at different life stages
- Predictive modeling for disease trajectory
- Pre-symptomatic intervention before disease develops
- Genome-guided wellness optimization throughout life
$
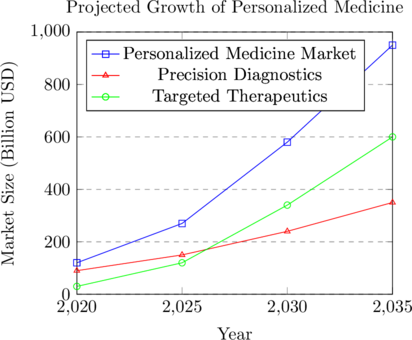
Global Health Applications
Extending personalized approaches worldwide:
- Affordable genetic testing for all populations
- Point-of-care precision diagnostics in resource-limited settings
- Adapted therapies accounting for global genetic diversity
- Infrastructure-appropriate implementations of precision medicine
Practical Applications and Case Studies
Clinical Success Stories
Cancer Precision Medicine Transformation
Targeted approaches changing cancer outcomes:
- Case example: HER2-positive breast cancer
- Traditional approach: General chemotherapy with poor outcomes
- Precision approach: HER2-targeted therapies (trastuzumab, pertuzumab)
- Outcome transformation: 5-year survival improved from <35% to >90%
- Ongoing innovation: Antibody-drug conjugates, dual HER2 targeting
Rare Disease Diagnosis Revolution
Ending diagnostic odysseys through genomics:
- Patient story: Undiagnosed neurological condition
- Traditional journey: Years of specialist visits, invasive tests, misdiagnoses
- Genomic approach: Whole genome sequencing identifying novel variant
- Treatment impact: Targeted intervention based on molecular mechanism
- Family benefit: Cascade testing for relatives at risk
Pharmacogenomic Implementation Success
Medication matching to genetic profiles:
- Hospital system example: Pre-emptive PGx testing program
- Implementation approach: Testing panel of high-impact genes
- Clinical integration: Decision support embedded in electronic records
- Results: 30% reduction in adverse events, improved efficacy
- Economic impact: Cost savings exceeding program implementation
Industry Transformations
Pharmaceutical R&D Reinvention
New development models for targeted therapies:
- Company case study: Precision pipeline transformation
- Traditional model: Broad target drugs, high failure rates
- Biomarker-driven approach: Patient selection from discovery phase
- Development impact: Higher success rates, smaller/faster trials
- Market outcomes: Premium pricing for high-efficacy, targeted drugs
Diagnostic Company Evolution
From general testing to precision approaches:
- Business transformation example: From pathology to molecular diagnostics
- Technology pivot: Investment in next-generation sequencing
- Service model: Integrated testing and interpretation
- Healthcare partnership: Clinician decision support integration
- Reimbursement strategy: Outcomes-based payment models
Patient Experience Transformations
Personalized Treatment Journey
Modern precision medicine experience:
- Patient pathway: Modern cancer care
- Initial approach: Comprehensive molecular profiling at diagnosis
- Treatment selection: Matching therapy to specific alterations
- Monitoring: Liquid biopsy tracking of treatment response
- Adaptation: Real-time adjustment based on emerging resistance
- Outcomes: Improved survival with better quality of life
Consumer Genomics to Clinical Care
Integration of direct-to-consumer testing:
- Patient example: From ancestry test to clinical intervention
- Initial engagement: Consumer curiosity about heritage
- Unexpected finding: Identification of disease risk variant
- Clinical transition: Confirmation through medical testing
- Preventive impact: Early intervention preventing disease
Frequently Asked Questions
What is the difference between personalized medicine and traditional medicine?
Personalized medicine and traditional medicine represent fundamentally different approaches to healthcare:
$
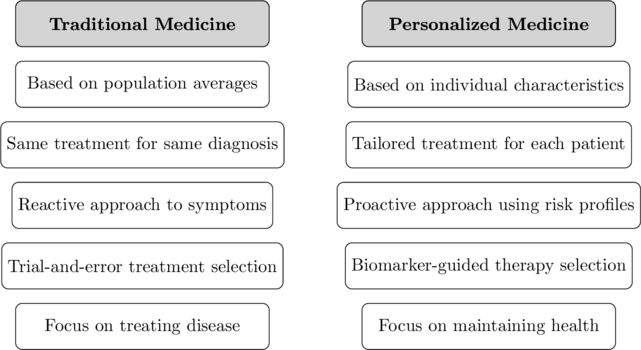
Traditional medicine typically:
- Applies a “one-size-fits-all” approach based on population averages
- Categorizes diseases by symptoms or affected organs
- Follows standardized treatment protocols for broad disease categories
- Often requires trying multiple treatments to find an effective option
- Focuses primarily on treating disease after it occurs
Personalized medicine instead:
- Tailors healthcare to individual genetic, environmental, and lifestyle factors
- Categorizes diseases by molecular mechanisms and pathways
- Selects treatments matched to specific biological characteristics
- Uses biomarkers to identify optimal treatment before administration
- Emphasizes prediction and prevention based on individual risk factors
The transition between these approaches is gradual, with many current medical practices incorporating elements of both traditional and personalized approaches.
How is genetic testing used in personalized medicine?
Genetic testing forms the foundation of many personalized medicine approaches and is used at multiple points in healthcare:
Risk Assessment and Prevention
- Hereditary cancer screening: Identifying inherited mutations in genes like BRCA1/2
- Cardiovascular risk profiling: Detecting genetic factors for heart disease
- Carrier testing: Determining risk of passing genetic conditions to children
- Polygenic risk scores: Assessing combined impact of many genetic variants
Diagnosis and Disease Classification
- Molecular diagnosis: Identifying specific genetic causes of symptoms
- Cancer subtyping: Classifying tumors based on genetic alterations
- Rare disease identification: Diagnosing uncommon genetic conditions
- Pathogen genotyping: Characterizing infectious agents
Treatment Selection and Optimization
- Pharmacogenomic testing: Predicting medication response and side effects
- Targeted therapy matching: Identifying patients for specific cancer drugs
- Dosing guidance: Optimizing medication amounts based on metabolism genes
- Resistance monitoring: Detecting genetic changes affecting treatment efficacy
Disease Monitoring
- Minimal residual disease detection: Finding trace amounts of cancer
- Transplant rejection surveillance: Monitoring donor-derived DNA
- Disease progression tracking: Following genetic markers of advancement
- Recurrence early warning: Detecting disease return before symptoms
The specific tests used range from targeted panels examining a few genes to comprehensive whole genome sequencing, with selection based on the clinical question and required detail.
Is personalized medicine covered by insurance?
Insurance coverage for personalized medicine varies widely depending on several factors:
- Test or treatment type: Some well-established approaches are routinely covered while newer technologies may not be
- Clinical evidence level: Stronger evidence of clinical utility increases coverage likelihood
- Regulatory approval status: FDA-approved tests and treatments more often covered
- Insurance provider policies: Coverage varies significantly between insurers
- Country and healthcare system: Dramatic differences in global coverage approaches
Current coverage trends include:
Generally Well-Covered
- Pharmacogenomic testing for certain medications (e.g., clopidogrel, warfarin)
- Companion diagnostic tests required for specific FDA-approved targeted therapies
- Cancer molecular profiling for advanced disease in many plans
- Carrier screening for reproductive planning
- Hereditary cancer testing with strong family history or risk factors
Variably Covered
- Broader genomic sequencing for rare disease diagnosis
- Tumor sequencing in early-stage cancer
- Polygenic risk scores for common diseases
- Liquid biopsy testing for cancer monitoring
- Pharmacogenomic testing for medications without FDA guidance
Rarely Covered
- Direct-to-consumer test confirmation
- Whole genome sequencing for healthy individuals
- Experimental precision therapies outside clinical trials
- Extensive molecular testing without clear medical necessity
Coverage is evolving rapidly as evidence accumulates, costs decrease, and clinical utility improves. Many health systems and insurers are developing specific policies for personalized medicine approaches.
What are the limitations of personalized medicine?
Despite its promise, personalized medicine faces several important limitations:
Biological Complexities
Challenges in understanding human biology:
- Multifactorial nature of most common diseases
- Gene-environment interactions difficult to measure
- Epigenetic influences changing gene expression
- Limited understanding of many genetic variants
- Phenotypic heterogeneity in genetically similar individuals
Technical Challenges
Issues with current technologies:
- Analytical validity varies between testing methods
- Interpretation complexity for genomic findings
- Data storage and management requirements
- Standardization lacking across platforms and labs
- Incidental findings creating interpretation dilemmas
Implementation Barriers
Difficulties in healthcare integration:
- Provider education gaps in genetics and genomics
- Health IT infrastructure inadequacy for complex data
- Clinical workflow disruption with new approaches
- Regulatory frameworks struggling to keep pace
- Reimbursement models not aligned with precision approaches
Ethical and Social Considerations
Broader societal challenges:
- Health disparities potentially exacerbated
- Privacy concerns with genetic information
- Psychological impacts of risk information
- Resource allocation questions for costly therapies
- Potential genetic discrimination despite legal protections
These limitations do not diminish the value of personalized medicine but highlight areas requiring continued research, policy development, and healthcare system adaptation.
How can I access personalized medicine approaches?
Accessing personalized medicine depends on your specific health needs and location, but several pathways exist:
Through Healthcare Providers
- Primary care physicians increasingly incorporate some personalized approaches
- Specialist referrals to genetics professionals or precision medicine programs
- Academic medical centers often lead in implementing advanced approaches
- Cancer centers frequently offer molecular profiling and targeted therapies
Clinical Testing Options
- Diagnostic genetic testing ordered by healthcare providers
- Pharmacogenomic testing through physicians or some pharmacies
- Carrier screening during family planning
- Tumor profiling as part of cancer care
Research Participation
- Clinical trials testing new precision therapies
- Research programs like All of Us (US) or 100,000 Genomes (UK)
- Biobanking initiatives combining samples and health information
- Hospital-based precision medicine programs collecting data while providing care
Consumer-Initiated Testing
- Direct-to-consumer genetic testing (with limitations)
- Health and wellness genomics through commercial providers
- Concierge medicine practices offering personalized health programs
- Online second opinion services with molecular interpretation
Steps to explore personalized medicine options:
- Discuss with healthcare providers your interest in precision approaches
- Research specific conditions for available personalized options
- Check insurance coverage before pursuing testing
- Consider genetic counseling when pursuing genetic testing
- Explore reputable resources like the Personalized Medicine Coalition or National Human Genome Research Institute
Conclusion: The Transformative Promise of Personalized Medicine
Personalized medicine represents one of the most significant paradigm shifts in healthcare history—moving from standardized, reactive approaches to individualized, proactive strategies based on each person’s unique biology. Through the powerful tools of biotechnology and genetic engineering, we are gaining unprecedented insights into the molecular basis of health and disease, enabling more precise prevention, diagnosis, and treatment.
The journey toward truly personalized healthcare involves numerous scientific, technical, ethical, and societal challenges. Yet the potential benefits—more effective treatments, fewer adverse effects, earlier disease detection, and eventually, disease prevention—offer compelling reasons to navigate these complexities.
As personalized medicine continues to evolve, its integration into routine healthcare will accelerate. What began in specialized fields like oncology and rare disease management is gradually expanding to chronic disease care, preventive medicine, and general health optimization. The convergence of genomics with digital health, artificial intelligence, and other emerging technologies promises to further enhance our ability to deliver the right care to the right patient at the right time.
The ultimate vision of personalized medicine extends beyond treating illness to maintaining wellness—a healthcare system focused on keeping individuals healthy based on their specific biology, environment, and lifestyle. While full realization of this vision remains on the horizon, each advancement in biotechnology and genetic engineering brings us closer to this transformative future.
Call to Action
To engage with the evolving field of personalized medicine:
- Discuss family health history with relatives to understand potential genetic risks
- Consider appropriate genetic testing in consultation with healthcare providers
- Ask about precision medicine options when facing significant health decisions
- Stay informed about advances through reputable sources like the National Human Genome Research Institute
- Participate in research if interested in contributing to medical knowledge advancement
- Advocate for policies that promote equitable access to personalized medicine
- Engage in conversations about the ethical implications of these technologies
The future of personalized medicine will be shaped not just by scientific breakthroughs but by how society collectively chooses to implement these powerful capabilities. By understanding both the potential and the challenges, we can help guide the development of personalized medicine toward its most beneficial and equitable applications.
<!– Schema Markup for SEO –> <script type=”application/ld+json”> { “@context”: “https://schema.org”, “@type”: “Article”, “headline”: “Personalized Medicine: The Future of Biotechnology and Genetic Engineering”, “description”: “A comprehensive guide to personalized medicine and its revolutionary impact on healthcare, covering technological breakthroughs, clinical applications, ethical considerations, and future trends.”, “image”: “https://example.com/images/personalized-medicine.jpg”, “author”: { “@type”: “Organization”, “name”: “Research.Help” }, “publisher”: { “@type”: “Organization”, “name”: “Research.Help”, “logo”: { “@type”: “ImageObject”, “url”: “https://example.com/logo.jpg” } }, “datePublished”: “2025-03-12”, “dateModified”: “2025-03-12” } </script> <!– Image Optimization Notes –> <!– Main Featured Image: – Filename: personalized-medicine-concept.jpg – Alt text: “Personalized medicine concept showing DNA analysis and tailored treatment approaches” – Size: 1200x630px for optimal social sharing – Format: WebP with JPEG fallback – Compress to <100KB without quality loss Section Images: 1. Genomic Testing Image – Alt text: “Doctor analyzing genomic sequencing results for personalized treatment plan” 2. Pharmacogenomics Image – Alt text: “Pharmacogenomic testing showing how genetic variations affect medication response” 3. Cancer Precision Medicine Image – Alt text: “Targeted cancer therapy based on tumor genetic profile analysis” 4. Future of Medicine Image – Alt text: “Integration of AI, genomics, and digital health in personalized medicine” –>









